|
UNITED STATES |
Washington, D.C. 20549
Form 6-K
Report of Foreign Private Issuer
Pursuant to Rule 13a-16 or 15d-16 under the Securities Exchange Act of 1934
For the month of January 2020
Commission File Number 001-37626
Mesoblast Limited
(Exact name of Registrant as specified in its charter)
Not Applicable
(Translation of Registrant’s name into English)
Australia
(Jurisdiction of incorporation or organization)
Silviu Itescu
Chief Executive Officer and Executive Director
Level 38
55 Collins Street
Melbourne 3000
Australia
(Address of principal executive offices)
Indicate by check mark whether the registrant files or will file annual reports under cover Form 20-F or Form 40-F:
Form 20-F ☑ Form 40-F ☐
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(1):
Yes ☐ No ☑
Indicate by check mark if the registrant is submitting the Form 6-K in paper as permitted by Regulation S-T Rule 101(b)(7):
Yes ☐ No ☑
INFORMATION CONTAINED ON THIS REPORT ON FORM 6-K
On January 15, 2020, Mesoblast Limited filed with the Australian Securities Exchange a new release announcement and presentation, which are attached hereto as Exhibit 99.1 and Exhibit 99.2, and are incorporated herein by reference.
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned, thereunto duly organized.
|
|
|
|
|
|
|
|
|
Mesoblast Limited |
|
|
|
|
|
/s/ Charlie Harrison |
|
|
|
|
|
|
|
|
|
|
|
Charlie Harrison |
|
|
|
|
|
Company Secretary
|
|
|
Dated: January 22, 2020
|
|
|
|
|
Item |
|
|
|
|
|
|
|
99.1 |
|
Press release of Mesoblast Ltd, dated January 15, 2020. |
|
99.2 |
|
Presentation of Mesoblast Ltd, dated January 15, 2020. |
Exhibit 99.1

MESOBLAST PRESENTS COMMERCIAL PLANS AT 2020 BIOTECH SHOWCASE IN SAN FRANCISCO
Announces Ryoncil™ as Brand Name for First Planned US Product Launch
Melbourne, Australia; January 15 and New York, USA; January 14, 2020: Mesoblast Limited (ASX:MSB; Nasdaq:MESO), global leader in allogeneic cellular medicines for inflammatory diseases, today reported that the United States Food and Drug Administration (US FDA) has agreed to the selection of Ryoncil™ as the commercial name for its lead allogeneic cell therapy remestemcel-L in the treatment of pediatric steroid-refractory acute graft versus host disease (aGVHD). Commercial plans for Ryoncil™ were presented at the 2020 Biotech Showcase being held this week in San Francisco, CA.
Mesoblast Chief Executive Dr Silviu Itescu said: “We begin 2020 with great excitement as we prepare for potential FDA approval and US launch of our lead product candidate Ryoncil™ in pediatric aGVHD, a potentially life-threatening complication of an allogeneic bone marrow transplant. The continued growth in revenues from royalties on sales in Japan of the related product TEMCELL®1 for aGVHD by our licensee provides important insight for our own US commercial plans. Together with our strategic partners, we are also looking forward to readouts of Phase 3 trials for our blockbuster product candidates in advanced chronic heart failure and chronic low back pain due to degenerative disc disease.”
The final module of the rolling Biologics License Application for Ryoncil™ will be filed with the FDA in January, following which the Company will request a priority FDA review of the BLA under the product candidate’s existing Fast Track designation. If approved, Ryoncil™ is planned to be launched in the US in 2020.
A webcast of the presentation is available via https://event.webcasts.com/starthere.jsp?ei=1278810&tp_key=f1494febd2 and as an archived webcast for 90 days on the Investors & Media section of the Company’s website at www.mesoblast.com.
About Ryoncil™ (remestemcel-L)
Mesoblast’s lead product candidate, Ryoncil™, is an investigational therapy comprising culture-expanded mesenchymal stem cells derived from the bone marrow of an unrelated donor. It is administered to patients in a series of intravenous infusions. Ryoncil™ is believed to have immunomodulatory properties to counteract the inflammatory processes that are implicated in aGVHD by down-regulating the production of pro-inflammatory cytokines, increasing production of anti-inflammatory cytokines, and enabling recruitment of naturally occurring anti-inflammatory cells to involved tissues.
About Mesoblast
Mesoblast Limited (ASX:MSB; Nasdaq:MESO) is a world leader in developing allogeneic (off-the-shelf) cellular medicines. The Company has leveraged its proprietary cell therapy technology platform to establish a broad portfolio of commercial products and late-stage product candidates. Two products have been commercialized in Japan and Europe by its licensees, and it has established commercial partnerships in Europe and China for certain Phase 3 assets. In the United States, Mesoblast has initiated submission of a rolling Biologics License Application to the FDA to seek approval of its product candidate for acute graft versus host disease following a successful Phase 3 trial, and is completing
|
|
Mesoblast Limited
www.mesoblast.com |
Corporate Headquarters Level 38 55 Collins Street Melbourne 3000 Victoria Australia
T +61 3 9639 6036 F +61 3 9639 6030 |
United States Operations 505 Fifth Avenue Third Floor New York, NY 10017 USA
T +1 212 880 2060 F +1 212 880 2061 |
Asia 21 Biopolis Road #01-22 Nucleos (South Tower) SINGAPORE 138567
T +65 6570 0635 F +65 6570 0176 |
|
|
|
|
|
|
|
|
Phase 3 trials for its advanced heart failure and chronic low back pain product candidates. Mesoblast’s proprietary manufacturing process yields industrial-scale, frozen, off-the-shelf, cellular medicines based on its mesenchymal lineage cell platform technology. These cell therapies, with pharmaceutical release criteria, are planned to be readily available to patients worldwide. Mesoblast has locations in Melbourne, New York, Singapore and Texas and is listed on the Australian Securities Exchange (MSB) and on the Nasdaq (MESO). For more information, please see www.mesoblast.com, LinkedIn: Mesoblast Limited and Twitter: @Mesoblast
Mesoblast’s Forward-Looking Statements
This announcement includes forward-looking statements that relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to differ materially from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. We make such forward-looking statements pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. Forward-looking statements should not be read as a guarantee of future performance or results, and actual results may differ from the results anticipated in these forward-looking statements, and the differences may be material and adverse. Forward-looking statements include, but are not limited to, statements about the timing, progress and results of Mesoblast’s preclinical and clinical studies; Mesoblast’s ability to advance product candidates into, enroll and successfully complete, clinical studies; the timing or likelihood of regulatory filings and approvals; and the pricing and reimbursement of Mesoblast’s product candidates, if approved. You should read this press release together with our risk factors, in our most recently filed reports with the SEC or on our website. Uncertainties and risks that may cause Mesoblast’s actual results, performance or achievements to be materially different from those which may be expressed or implied by such statements, and accordingly, you should not place undue reliance on these forward-looking statements. We do not undertake any obligations to publicly update or revise any forward-looking statements, whether as a result of new information, future developments or otherwise.
Release authorized by the Chief Executive.
1.TEMCELL HS Inj. is a registered trademark of JCR Pharmaceuticals Co. Ltd.
For further information, please contact:
Julie Meldrum
Corporate Communications
T: +61 3 9639 6036
E: julie.meldrum@mesoblast.com
Schond Greenway
Investor Relations
T: +1 212 880 2060
E: schond.greenway@mesoblast.com
|
|
Mesoblast Limited
www.mesoblast.com |
Corporate Headquarters Level 38 55 Collins Street Melbourne 3000 Victoria Australia
T +61 3 9639 6036 F +61 3 9639 6030 |
United States Operations 505 Fifth Avenue Third Floor New York, NY 10017 USA
T +1 212 880 2060 F +1 212 880 2061 |
Asia 21 Biopolis Road #01-22 Nucleos (South Tower) SINGAPORE 138567
T +65 6570 0635 F +65 6570 0176 |
|
|
|
|
|
|
|
|

ASX: MSB; Nasdaq: MESO Corporate Overview Dr Silviu Itescu, Chief Executive January 2020 Exhibit 99.2

CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS This presentation includes forward-looking statements that relate to future events or our future financial performance and involve known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, performance or achievements to differ materially from any future results, levels of activity, performance or achievements expressed or implied by these forward-looking statements. We make such forward-looking statements pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995 and other federal securities laws. All statements other than statements of historical facts contained in this presentation are forward-looking statements. Words such as, but not limited to, “believe,” “expect,” “anticipate,” “estimate,” “intend,” “plan,” “targets,” “likely,” “will,” “would,” “could,” and similar expressions or phrases identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and future events , recent changes in regulatory laws, and financial trends that we believe may affect our financial condition, results of operation, business strategy and financial needs. These statements may relate to, but are not limited to: expectations regarding the safety or efficacy of, or potential applications for, Mesoblast's adult stem cell technologies; expectations regarding the strength of Mesoblast's intellectual property, the timeline for Mesoblast's regulatory approval process, and the scalability and efficiency of manufacturing processes; expectations about Mesoblast's ability to grow its business and statements regarding its relationships with current and potential future business partners and future benefits of those relationships; statements concerning Mesoblast's share price or potential market capitalization; and statements concerning Mesoblast's capital requirements and ability to raise future capital, among others. Forward-looking statements should not be read as a guarantee of future performance or results, and actual results may differ from the results anticipated in these forward-looking statements, and the differences may be material and adverse. You should read this presentation together with our financial statements and the notes related thereto, as well as the risk factors, in our most recently filed reports with the SEC or on our website. Uncertainties and risks that may cause Mesoblast's actual results, performance or achievements to be materially different from those which may be expressed or implied by such statements, include, without limitation: risks inherent in the development and commercialization of potential products; uncertainty of clinical trial results or regulatory approvals or clearances; government regulation; the need for future capital; dependence upon collaborators; and protection of our intellectual property rights, among others. Accordingly, you should not place undue reliance on these forward-looking statements. We do not undertake any obligations to publicly update or revise any forward-looking statements, whether as a result of new information, future developments or otherwise. |

Our Mission Mesoblast is committed to bringing to market innovative cellular medicines to treat serious and life-threatening illnesses |

2004: Mesoblast founded in Melbourne, Australia and listed on the ASX 2014: Granted manufacturing pioneer status by Economic Development Board of Singapore 2013: Acquired MSC business from Osiris Therapeutics with future earn-outs 2011: Entered into manufacturing partnership with Lonza Group in Singapore for MPC medicines 2015: Dual listed on the Nasdaq 2016: TEMCELL® HS Inj (MSC medicine) launched in Japan by Mesoblast licensee JCR 2017: Entered licensing agreement with Takeda for the treatment of certain fistulae; in 2018 Alofisel® received approval in EU 2018: Entered into strategic partnership agreement with Tasly for cardiovascular assets in China 2019: Initiated first BLA submission to US FDA: remestemcel-L (MSC) for steroid refractory acute graft versus host disease (aGVHD) Corporate History 2010: Entered into strategic alliance with Cephalon to develop and commercialize MPC therapeutics 2019: Smith & Nephew acquired Osiris Therapeutics, and will receive future earn-outs on MSC business Over a decade of scientific, manufacturing, clinical development and corporate development experience targeted at bringing to market allogeneic, off-the-shelf cellular medicines for inflammatory diseases 2019: Entered into strategic partnership with Grünenthal for chronic low back pain asset in Europe & Latin America |

Premier Global Cellular Medicines Company Commercialization Innovative Technology Platform1 Innovative technology targets some of the most severe disease states refractory to conventional therapies Well characterized multimodal mechanisms of action Underpinned by extensive, global IP estate Mesenchymal precursor cells (MPCs) and their culture-expanded progeny mesenchymal stem cells (MSCs). Licensee JCR Pharmaceuticals Co., Ltd. received the first full PMDA approval for an allogeneic cellular medicine in Japan and markets this product under its trademark, TEMCELL® Hs Inj. Licensee Takeda received first central marketing authorization approval from the European Commission for an allogeneic stem cell therapy and markets this product under its trademark Alofisel®. Late Stage Pipeline Initiated rolling filing with US FDA for approval for steroid-refractory aGVHD Two Phase 3 product candidates – heart failure and back pain – with near term US trial readouts Back pain Phase 3 product candidate partnered in Europe & Latin America with Grünenthal Heart failure Phase 3 product candidate partnered in China Building US sales force for potential aGVHD product launch Industrial-scale manufacturing to meet commercial demand First approved products commercialized by licensees in Japan2 and Europe3 Continued growth in royalty revenues from strategic partnerships |

Commercial Scale Manufacturing Capability Scalable allogeneic “off-the-shelf” cellular medicine platform Manufacturing meets stringent criteria set by international regulatory agencies including FDA and EMA Robust quality assurance processes ensure final product with batch-to-batch consistency and reproducibility Culture expansion scalable for near term commercial needs Proprietary xeno-free technologies being developed to enable sufficient yields for long term global commercial supply Next generation processes using 3D bioreactors to reduce labor and drive down cost of goods Lonza contract manufacturing facility in Singapore |

Markets U.S., Europe, China, and Japan Diseases All Tier 1 & Tier 2 Indications, and multiple additional conditions Sources Allogeneic, Autologous, (Bone Marrow, Adipose, Dental Pulp, Placenta), Pluripotent (iPS) ~1,000 patents and patent applications (68 patent families) across all major jurisdictions Covers composition of matter, manufacturing, and therapeutic applications of mesenchymal lineage cells Enables licensing to third parties for different indications, when in alignment with our corporate strategy e.g.TiGenix (subsequently acquired by Takeda) Provides strong global protection against competitors seeking to develop products in areas of core commercial focus Mesenchymal Lineage Precursors and Progeny Global IP Estate Provides Substantial Competitive Advantage |

IN DEVELOPMENT PLATFORM PRODUCT THERAPEUTIC AREA APPROVAL COMMERCIAL RIGHTS MSC TEMCELL® HS Inj1 Acute Graft Versus Host Disease Japan MSC Alofisel®2 Perianal Fistula Global MARKETED Commercial and Late-Stage Product Pipeline This chart is figurative and does not purport to show individual trial progress within a clinical program PLATFORM PRODUCT CANDIDATE THERAPEUTIC AREA PRE-CLINICAL PHASE 2 PHASE 3 COMMERCIAL RIGHTS MSC (Remestemcel-L) Ryoncil® Acute Graft Versus Host Disease Crohn’s Disease RevascorTM Advanced HF (Class II-IV) End-Stage HF China ROW MPC (Rexlemestrocel) MPC-06-ID Chronic Low Back Pain Europe Lat Am ROW MPC-300-IV Rheumatoid Arthritis Diabetic Nephropathy 1st allogeneic regen med approved in Japan 1st allogeneic regen med approved in Europe BLA submission to FDA underway TEMCELL® Hs. Inj. is a registered trademark of JCR Pharmaceuticals Co Ltd Alofisel® is a registered trademark of Takeda Pharmaceuticals

Strategic partnership to develop and commercialize MPC-06-ID for chronic low back pain due to degenerative disc disease in patients who have exhausted conservative treatment options Grünenthal will have exclusive commercialization rights for Europe and Latin America Mesoblast will receive up to US$150 million in upfront and milestone payments prior to product launch, as well as further commercialization milestone payments Cumulative milestone payments could exceed US$1 billion depending on the final outcome of Phase III studies and patient adoption. Mesoblast will also receive tiered double digit royalties on product sales JCR has rights to use our MSC technology to treat acute GVHD in Japan Its product TEMCELL® HS Inj. was the first fully approved allogeneic cellular medicine in Japan Royalties and milestones received in last 12 months exceed US$6.0 million License expanded to cover use in epidermolysis bullosa (EB), a highly debilitating and sometimes lethal skin disease and hypoxic ischemic encephalopathy (HIE) in newborns Patent license agreement entered in Dec 2017 with Takeda (formerly TiGenix NV) providing exclusive access to certain IP for local treatment of perianal fistulae Mesoblast received €10 million in payments and is eligible to receive up to an additional €10 million in milestone payments (€20 million in total payments) plus royalties upon commercial sales of Alofisel® worldwide Exclusive cardiovascular rights in China Mesoblast received US$40 million on closing, and is eligible to receive additional milestones and royalties Partnerships and License Agreements |

Overview of Lead Product Candidates

Minimal Treatment Options Market Opportunity Burden of Illness aGVHD is a life-threatening complication that occurs in ~50% of patients receiving allogeneic bone marrow transplants (BMTs)1 Steroid-refractory aGVHD is associated with mortality rates as high as 90%1,7 and significant extended hospital stay costs2 There is only one approved treatment for SR-GVHD and no approved treatment for children under 12 years old, outside Japan In Japan, Mesoblast’s licensee has received the only product approval for SR - aGVHD in both children and adults >30,000 allogeneic BMTs performed globally (>20K US/EU) annually, ~20% pediatric3,4 Our licensee, JCR Pharmaceuticals Co., Ltd launched TEMCELL® HS Inj.5 in Japan for SR- aGVHD in 2016; reimbursed up to ~$USD195k6 SR-aGVHD represents $USD > 700m US/EU market opportunity4,8 Acute Graft Versus Host Disease (aGVHD) Significant market opportunity for remestemcel-L 1. Westin, J., Saliba, RM., Lima, M. (2011) Steroid-refractory acute GVHD: predictors and outcomes. Advances in Hematology. 2. Anthem-HealthCore/Mesoblast claims analysis (2016). Data on file 3. Niederwieser D, Baldomero H, Szer J. (2016) Hematopoietic stem cell transplantation activity worldwide in 2012 and a SWOT analysis of the Worldwide Network for Blood and Marrow Transplantation Group including the global survey. 4. Source: CIBMTR Current Uses and Outcomes of Hematopoietic Cell Transplantation 2017 Summary. Passweg JR, Baldomero, H (2016) Hematopoietic stem cell transplantation in Europe 2014: more than 40,000 transplants annually. 5. TEMCELL is the registered trademark of JCR Pharmaceuticals Co. Ltd. 6. Based on a ¥JPY = $USD 0.009375 spot exchange rate on market close on November 11, 2016. Amounts are rounded. Source: Bloomberg. 7. Axt L, Naumann A, Toennies J (2019) Retrospective single center analysis of outcome, risk factors and therapy in steroid refractory graft-versus-host disease after allogeneic hematopoietic cell transplantion. Bone Marrow Transplantation. 8.Data on file |

Grade C/D GVHD has Significantly Worse Survival than Grade A/B | IBMTR severity grade Glucksberg severity grade

65% 1.Kurtzberg et al: Presentation Tandem Feb 2016 73% 67% 61% 68% 65% 62% 0% 20% 40% 60% 80% 100% All Patients Grade BGrade CGradeD (n=241)(n=48)(n=73)(n=120) Any SkinAny GIAny Liver (n=114)(n=208)(n=66) Complete Response was 14%, Partial Response was 51% Responses were observed for all GVHD grades and did not differ by baseline organ involvement Overall Response at Day 28 in 241 pediatric aGVHD patients receiving remestemcel-L as salvage therapy1 241 pediatric patients undergoing HSCT were enrolled and treated at 50 sites in North America and Europe from 2007-2014 Ages 2 months – 17 years Grade C/D in 80% of patients Failed steroid treatment and multiple other agents aGVHD not improving after at least 3 days of methylprednisolone (at least 1 mg/kg/day or equivalent) Remestemcel-L: Expanded Access Program (Protocol 275) Remestemcel-L used as salvage therapy in children who have failed steroids and other agents in treatment of aGVHD, including the most severe Grade C/D disease Response Rate |
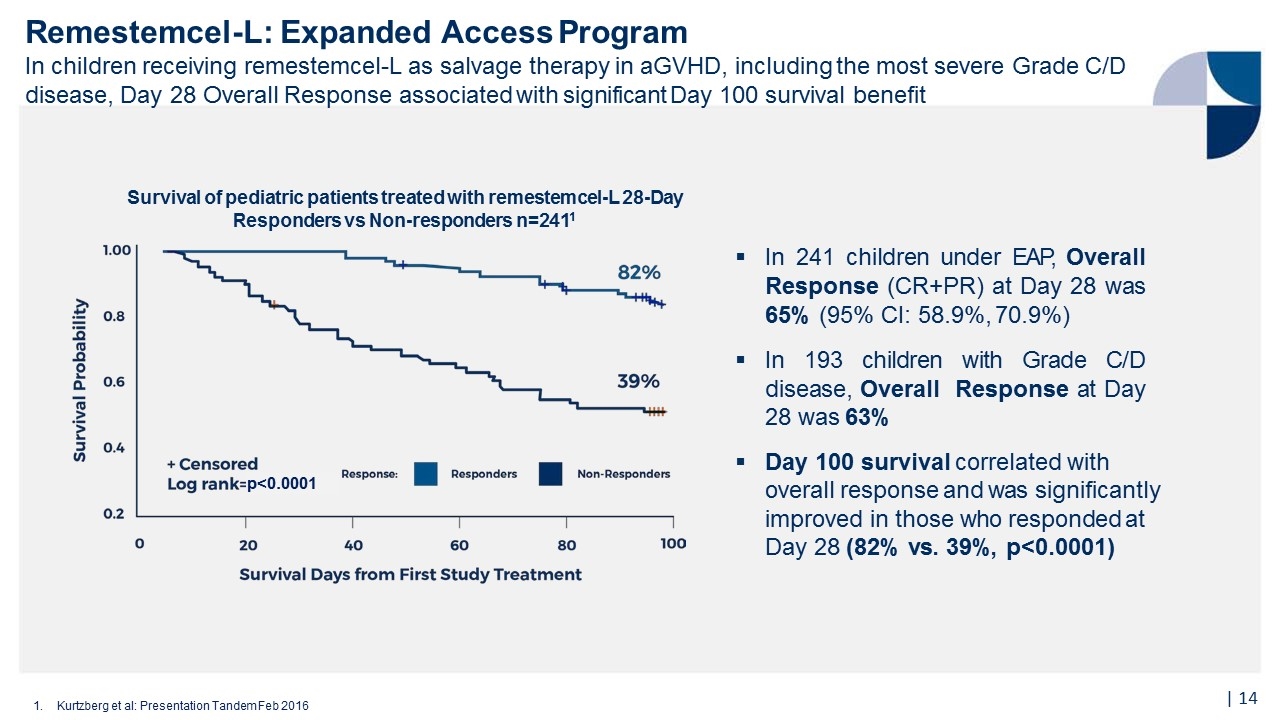
Remestemcel-L: Expanded Access Program In children receiving remestemcel-L as salvage therapy in aGVHD, including the most severe Grade C/D disease, Day 28 Overall Response associated with significant Day 100 survival benefit 1.Kurtzberg et al: Presentation Tandem Feb 2016 Survival of pediatric patients treated with remestemcel-L 28-Day Responders vs Non-responders n=2411 In 241 children under EAP, Overall Response (CR+PR) at Day 28 was 65% (95% CI: 58.9%, 70.9%) In 193 children with Grade C/D disease, Overall Response at Day 28 was 63% Day 100 survival correlated with overall response and was significantly improved in those who responded at Day 28 (82% vs. 39%, p<0.0001) p<0.0001 |
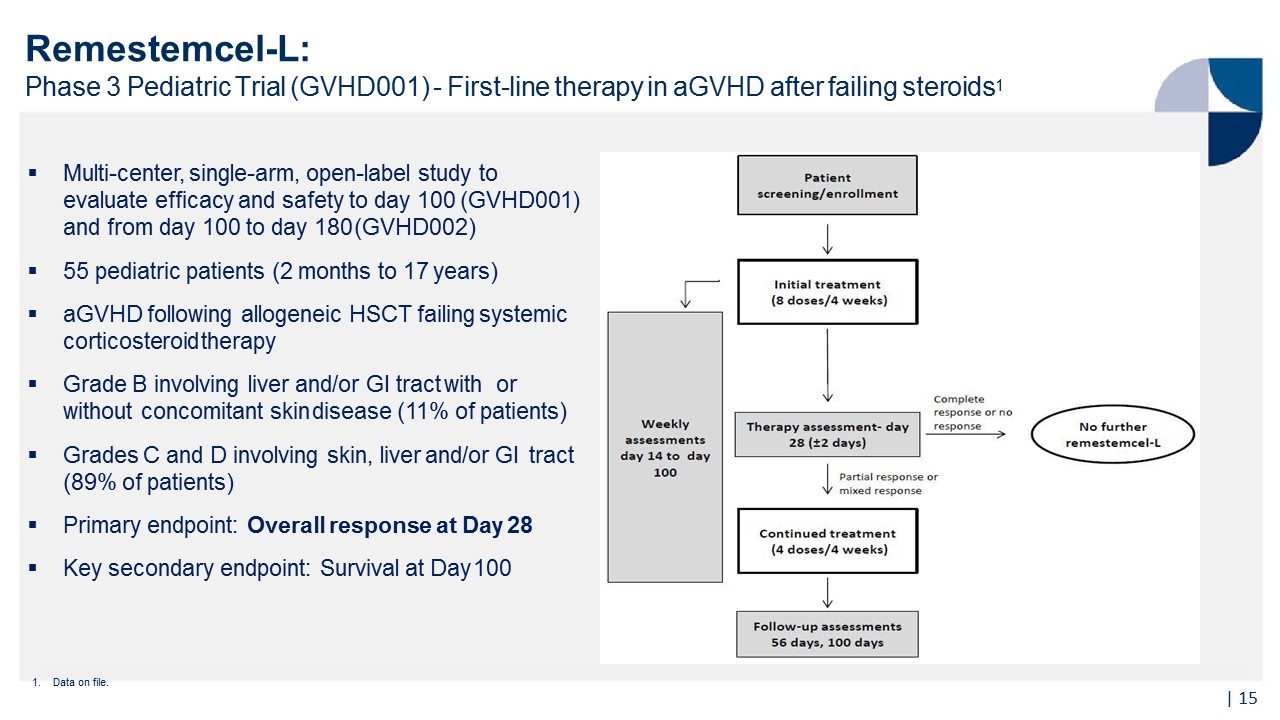
Multi-center, single-arm, open-label study to evaluate efficacy and safety to day 100 (GVHD001) and from day 100 to day 180 (GVHD002) 55 pediatric patients (2 months to 17 years) aGVHD following allogeneic HSCT failing systemic corticosteroid therapy Grade B involving liver and/or GI tract with or without concomitant skin disease (11% of patients) Grades C and D involving skin, liver and/or GI tract (89% of patients) Primary endpoint: Overall response at Day 28 Key secondary endpoint: Survival at Day 100 Remestemcel-L: Phase 3 Pediatric Trial (GVHD001) - First-line therapy in aGVHD after failing steroids1 1. Data on file. |

50% 70% 76% 70% 0% 20% 40% 60% 80% Grade B Grade CGrade D (n=6) (n=23)(n=25) All Subjects (n= 54) Data on file GVHD001 had 55 randomized patients, however one patient dropped out before receiving any dose of remestemcel-L Remestemcel-L: Phase 3 Trial Protocol GVHD001 - Primary efficacy overall response at Day 28 was 70%, p=0.00031,2 70% Overall Response rate at Day 28 (30% CR + 41% PR); (95% CI: 56%, 82%) p-value calculated from the binomial distribution, under the assumption of a 0.45 success rate under the null hypothesis 100% Response Rate |

Remestemcel-L: Protocol GVHD001/002 survival1 In children receiving remestemcel-L as first-line therapy in SR-aGVHD, including Grade C/D disease, Day 28 Overall Response associated with significant Day 100 survival benefit 1. Data on file. Overall Survival Probability Days Since First Dose of Remestemcel-L Day 100 87% | Day 180 79% Survival in Day 28 Responders Survival in Day 28 Non-Responders

Remestemcel-L: Phase 3 Trial1 Phase 3 study evaluated remestemcel-L in 55 children to improve overall response rate and survival 89% of children had grade C/D disease, the most severe form and historically associated with up to 90% mortality2,3 Study successfully met the primary endpoint of improved Day 28 Overall Response (OR) 70% vs 45% protocol-defined historical control rate (p=0.0003) Day 100 Overall Survival 74%, with 87% survival in Day 28 responders Day 180 Overall Survival 69%, with 79% survival in Day 28 responders Remestemcel-L infusions well tolerated Findings consistent with previous results in 241 SR-aGVHD children under expanded access program who failed to respond to multiple biologic agents4 Data on file. Westin, J., Saliba, RM., Lima, M. (2011) Steroid-refractory acute GVHD: predictors and outcomes. Advances in Hematology. Axt L, Naumann A, Toennies J (2019) Retrospective single center analysis of outcome, risk factors and therapy in steroid refractory graft-versus-host disease after allogeneic hematopoietic cell transplantation. Bone Marrow Transplantation Kurtzberg J. et al. Effect of Human Mesenchymal Stem Cells (remestemcel-L) on Clinical Response and Survival Confirmed in a Large Cohort of Pediatric Patients with Severe High-Risk Steroid-Refractory Acute Graft Versus Host Disease. BBMT. 2016; 22. |

Remestemcel-L: Phase 3 Trial compared to MAGIC Database Improved Day 28 Overall Response and Day 100 Survival relative to matched controls Outcomes* MSB-GVHD001 (n=54)2 MAGIC SR-aGVHD (n=30)3 Day 28 Overall Response 38 (70%) 13 (43%) Day 100 Survival 40 (74%) 16/28 (57%) Mount Sinai Acute GVHD International Consortium (MAGIC) - a group of ten BMT centers throughout the US and Europe whose purpose is to conduct ground-breaking clinical trials in GVHD, including developing informative biorepositories that assist in developing treatments that can guide GVHD therapy. GVHD001 had 55 randomized patients, however one patient dropped out before receiving any dose of remestemcel-L Two subjects in the MAGIC cohort had follow-up <100 days; these subjects are excluded from the respective survival analyses. *rounded to nearest % | A comparative analysis performed between Mesoblast’s open-label Phase 3 study and contemporaneous controls receiving institutional standard of care Phase 3 trial of remestemcel-L (GVHD001) in 55 children, 89% of whom had Grade C/D disease A cohort of 30 pediatric patients with SR-aGVHD from the MAGIC consortium matched for inclusion criteria and disease severity (80% Grade C/D) These results demonstrate the effectiveness of remestemcel-L in this patient population, with particular efficacy and survival benefit in patients with the most severe forms of aGVHD

Remestemcel-L: U.S. Regulatory and Commercial Strategy US strategy for remestemcel-L informed by TEMCELL sales experience in Japan Rolling BLA submission to FDA for treatment of pediatric SR-aGVHD Fast Track designation provides eligibility for FDA priority review Commercialization strategy in place for product launch Ramp-up for inventory build Building out efficient, targeted sales force - 15 centers account for ~50% of patients Label extension for treatment of adult SR-aGVHD Life-cycle extension to new indications |

37% growth in royalty revenue for the FY2019 year compared to FY2018 from sales of TEMCELL in Japan for SR-aGVHD by Mesoblast licensee JCR Pharmaceuticals Co. Ltd. Significant Annual Revenue Growth from Royalties on GVHD Sales in Japan All results on this slide are reported in constant currency. |

Remestemcel-L: Life Cycle Strategy 2013 Mesoblast acquires MSC platform from Osiris Therapeutics Product development/ manufacturing optimization 2016 JCR Pharmaceuticals (licensee) launches TEMCELL in Japan SR-aGVHD pediatric US launch planned 2020 SR-aGVHD pediatric Ex-US launch 2018 Pediatric Phase 3 (US) top line data SR-aGVHD adult US/Ex-US parallel launch Label extension Chronic GVHD Label extension Crohns & other indications |

Remestemcel-L for Acute GVHD Recent Highlights Continued growth in revenues from royalties on sales of TEMCELL in Japan for steroid refractory aGVHD Product adoption and reimbursement seen in the Japan GVHD market for TEMCELL informs Mesoblast US commercial strategy for remestemcel-L in aGVHD US addressable market for SR aGVHD in children and adults is expected to be approximately 8-fold larger than Japan, a major commercial opportunity due to greater patient numbers, incidence and pharmacoeconomics Mesoblast entered into an agreement with Lonza for commercial product manufacture in line with the corporate strategy to facilitate appropriate inventory build ahead of the planned launch of remestemcel-L Key milestones Final module of rolling Biologic License Application (BLA) submission scheduled for filing with the US Food and Drug Administration (FDA) in January 2020 Mesoblast will seek Priority Review by the FDA under the product candidate’s existing Fast Track designation If approved, the US launch of remestemcel-L is expected to occur in 2020 |

MPC-06-ID: A New Paradigm for Treatment of Chronic Low Back Pain Due to Degenerative Disc Disease | Minimal Treatment Options Market Opportunity Burden of Illness Unmet Need Back pain causes more disability than any other condition1 Inflicts substantial direct and indirect costs on the healthcare system1,2, including excessive use of opioids in this patient population Minimal treatment options for patients with chronic low back pain (CLBP) who fail conservative therapy include opioids and surgery 50% of opioid prescriptions are for CLBP Over 7m patients are estimated to suffer from CLBP due to degenerative disc disease (DDD) in each of the U.S. and E.U.5 3-6 MPC-06-ID development program targets over 3.2m patients in U.S. and 4m in E.U.5 with moderate to severe disease Williams, J., NG, Nawi, Pelzter, K. (2015) Risk factors and disability associated with low back pain in older adults in low-and middle-income countries. Results from the WHO Study on global ageing and adult health (SAGE). PloS One. 2015; 10(6): e0127880., 2. Simon, J., McAuliffe, M., Shamim, F. (2015) Discogenic Low Back Pain. Phys Med Rehabil Clin N Am 25 (2014) 305–317., 3.Decision Resources: Chronic Pain December 2015., 4. LEK & NCI opinion leader interviews, and secondary analysis., 5. Navigant: Commercial Assessment for a Proprietary Cell-Based Therapy for DDD in the U.S. and the EU3 – August 2014., 6. HealthCare Utilization and Cost of Discogenic Lower Back Pain in the US – Anthem/HealthCore. Disease modifying therapy for durable improvement in pain and function has potential to prevent progression to opioid use or surgical intervention

MPC-06-ID – Development Strategy for US & Europe Phase 3 trial in chronic low back pain completed enrolment in March 2018 with 404 patients randomized to receive MPC-06-ID or placebo Initiate confirmatory Phase 3 trial in Europe in partnership with Grünenthal Complete commercial manufacturing in partnership with Grünenthal Results of confirmatory Phase 3 clinical trials in US and Europe, together with commercial manufacturing, expected to support regulatory approval and commercial launches in both Europe and US for MPC-06-ID in chronic low back pain due to degenerative disc disease |

Key Terms of the Strategic Partnership with Grünenthal Grünenthal has obtained An exclusive license for Europe and Latin America to develop and commercialize MPC-06-ID in the treatment of chronic low back pain due to degenerative disc disease In consideration, Mesoblast will receive Up to US$150 million in upfront and milestone payments prior to product launch, as well as further commercialization milestone payments Payments include commitments up to US$45 million within the first year comprising US$15 million on signing, US$20 million on receiving regulatory approval to begin a confirmatory Phase 3 trial in Europe, and US$10 million on certain clinical and manufacturing outcomes Cumulative milestone payments could exceed US$1 billion depending on the final outcome of Phase 3 studies and patient adoption Mesoblast will also receive tiered double digit royalties on product sales Mesoblast retains the rights for the rest of world, including the US and Japan markets |

Transaction Benefits to Mesoblast Strong commercial partner Delivers commercialization, distribution, sales & marketing Field force comprises around 1,600 people across Europe, Latin America & US – overall focus is on pain – visited nearly 300,000 stakeholders in 2018 (physicians, pharmacists & health administrators) Provides knowledge and knowhow in manufacturing, regulatory affairs (Europe in particular) Advances approval pathway Provides funding for Phase 3 trial in Europe reducing Mesoblast cash outflow Mesoblast and Grünenthal will collaborate on the study design for a confirmatory Phase 3 trial in Europe Confirmatory European and US (currently ongoing) Phase 3 trials are expected to support regulatory approval in both Europe and US ü Transaction focuses on Europe Mesoblast maintains rights to all other geographic markets, including US, Japan and China for additional partnering opportunities to maximize shareholder return Third party endorsement provides validation of technology platform |

MPC-06-ID for Chronic Low Back Pain Key Milestones Last patient last visit at 24-months of follow up in the Phase 3 trial of MPC-06-ID for chronic low back pain H1 CY20, with the primary endpoint being a composite outcome of pain and function at 12 and 24 months Obtain clearance in 2020 from European regulatory authorities to begin European Phase 3 trial Results from the Phase 3 trials will be considered pivotal to support regulatory approval in the US, as well as Europe through the Grünenthal partnership |

Source: Simon-Kucher & Partners 2017. Primary research 2017; Payers n=35, KOLs n=15,Cath lab managers n=4. Corlanor® (ivabradine) approved by FDA (April 2015). ENTRESTO® (sacubitril/valsartan) approved by FDA (July 2015). GlobalData-PharmaPoint Heart Failure (2016); McMurray et al., 2012;Yancy et al., 2013, 2016 ACC/AHAHFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. Early ACEI or ARB Statins Beta blockers Re-vascularization or valvularsurgery Advanced End-Stage Pharmacological Add-on Diuretics for fluid retention Aldosterone antagonists Hydralazine / isosorbidedinitrate Digitalis New Oral Therapies for Class II-IV2 If ACEI / ARB tolerated, sacubitril/valsartan Common Treatment Pathway in Progressive Heart Failure1 Mesoblast Target Market: Advanced and End-Stage HF patients3 Cardiac Resynchronization Therapy (CRT) Implantable Cardioverter-Defibrillator (ICD) LVAD Heart transplants Limited Therapeutic Options Advanced and End-Stage Heart Failure Progressive Vascular (Endothelial) Dysfunction and Heart Failure Class I Class IV |

Market Opportunity Burden of Illness Limited Options / Unmet Need More than 26 million people worldwide living with heart failure1 8.5 million patients in the US alone, expected to be living with heart failure by 20301 17-45% of patients die within 1 year of hospital admission1 Advanced / End Stage heart failure defined as NYHA class III / IV and comprise ~30% of all HF patients2,3 Despite new therapies for early-stage disease, there has been very little improvement in survival for patients with advanced heart failure2,3 Majority of advanced heart failure patients die within 5 years1 In the US, more hospital days are spent on the care of patients with HF than any other diagnosis2,3 Advanced HF has the highest hospital readmission rate of any diagnosis-related group, indicative of the limited treatment options when patients reach this stage2,3 Large clinical unmet need with multi-billion dollar annual market opportunity in US4,5 1. Heart Failure: Preventing disease and death worldwide – European Society of Cardiology 2014. 2. Leslie W.MillerMD, Maya GuglinMD, PhD. Patient Selection for Ventricular Assist Devices: A Moving Target. Journal of the American College of Cardiology. Volume 61, Issue 12, 26 March 2013, Pages 1209-1221, 3. Leslie W. Miller. Left Ventricular Assist Devices Are Underutilized. Circulation. 2011;123:1552–1558. 4.A Reevaluation of the Costs of Heart Failure and its Implications for Allocation of Health Resources in the United States. Voigt J. Clinl.Cardiol. 37, 5, 312- 321 (2014)., 5.The Medical and Socioeconomic Burden of Heart Failure: A Comparative Delineation with Cancer. Dimitrios, F. International Journal of Cardiology (2015), doi: 10.1016/j.ijard.2015.10.172. Advanced & End-Stage Heart Failure Rising incidence of HF1 together with high mortality rates highlight large clinical unmet need |

Revascor Phase 2: Maximal Therapeutic Benefit on Left Ventricular Volumes Seen in Subjects with LVESV >100ml1 Source : Perin et al., Journal of Cardiac Failure 2015; Vol 21(8): S107; 19th Annual Scientific Meeting of the Heart Failure Society of America, Emerson et al. LVESV = Left ventricular end systolic volume; LVEDV = Left Ventricular End-Diastolic Volume; LVEF = Left Ventricular Ejection Fraction. Placebo (PBO) corrected benefit of 150MM cell dose on cardiac volumes and ejection fraction at 6 months was greatest in patients with more advanced heart failure as defined by baseline LVESV>100ml at baseline Change (Entire cohort) Month 6 minus baseline PBO (n=15) 150M MPC∆, PBO PBO (n=7) 150M MPC∆, PBO P-values (n=15) corrected (n=11) corrected LVESV +20 -7 -27 +46 -8 -54 <0.02 LVEDV +20 -10 -30 +41 -10 -51 <0.03 LVEF -2.3 +0.6 +2.9 -6.4 +1.7 +8.1 <0.05 Change (LVESV>100mL) Month 6 minus baseline ∆ ∆ ∆ ∆ ∆ ∆

Revascor Phase 2: A Single Dose Prevented Any Heart Failure-Related Major Adverse Cardiac Event (HF-MACE) for 36 Months in High Risk Patients % HF-MACE Kaplan-Meier Curve over 36 months following treatment in all patients1 HF-MACE Kaplan-Meier Curve over 36 months following treatment in patients with LVESV>100ml2 1. HF-MACE is defined as a composite of cardiac related death or non-fatal heart failure hospitalisations. 2. Circ Res. 2015; 117:576-584. Perin E et al. A Phase II Dose-Escalation Study of Allogeneic Mesenchymal Precursor Cells in Patients With Ischemic or Non-Ischemic Heart Failure 3. Journal of Cardiac Failure 2015; Vol 21(8): S107; 19th Annual Scientific Meeting of the Heart Failure Society of America, Emerson et al. Over 36 months, patients receiving 150M MPC had significantly greater probability of remaining free of a first HF-MACE vs. controls (0% vs. 33%, p = 0.026 by log-rank) All HF-MACE events occurred in controls with baseline Left Ventricular End Systolic Volume (LVESV)>100ml, where the treatment effect size was even greater (0% vs. 71%, p = 0.0007 by log rank) Controls with baseline LVESV>100ml had 11 total/recurrent HF-MACE events over 36 months vs. 0 in matched patients receiving 150M MPCs (p=0.0007) Time to HF-MACE (days)

Trial design: 1:1 randomized, controlled, double blinded; conducted over 55 sites across North America using 150 million cell dose vs control Target patient population enriched for those with LVESV>100ml, at highest risk for events and greatest responders to Revascor therapy Primary endpoint: reduction in recurrent heart failure-related major adverse cardiac events (HF-MACE) such as heart failure-related hospitalizations and cardiac death Secondary endpoint: reduction in terminal cardiac events In April 2017, a pre-specified interim futility analysis of the primary efficacy endpoint in the Phase 3 trial’s first 270 patients was successful Events-driven Phase 3 trial completed enrollment of 566 patients in February 2019 In December 2019, surpassed the number of primary endpoint events required for trial completion in the Phase 3 trial of Revascor for advanced heart failure Advanced Heart Failure Revascor - Phase 3 trial |

Revascor for Advanced Stage Heart Failure Key milestones Initiated final study visits for all surviving patients in Phase 3 trial of Revascor in Advanced Heart Failure, with a target of last patient/last visit at the end of January 2020 Data read-out for this Phase 3 trial expected by mid-2020 Results will be considered pivotal to support regulatory approvals Plans for commercial launch in conjunction with appropriate pharma partners in the US / EU / Japan, and in China through the Tasly partnership |

Revascor for End-Stage Heart Failure in LVAD Patients Recent Highlights Mesoblast and the International Center for Health Outcomes Innovation Research (InCHOIR) at the Icahn School of Medicine at Mount Sinai in New York have agreed on the protocol for a confirmatory Phase 3 trial of Revascor In line with FDA guidance, the primary endpoint will be reduction in major mucosal bleeding events, and key secondary endpoints will be improvement in various parameters of cardiovascular function Revascor is being developed for these patients under existing FDA Regenerative Medicine Advanced Therapy (RMAT) and Orphan Drug designations Key milestones Initiation of confirmatory Phase 3 trial of Revascor for the reduction of mucosal bleeding in end-stage heart failure patients implanted with an LVAD Results will be considered pivotal to support regulatory approval in the US |

Anticipated Major Milestones – Next 12 Months Remestemcel-L for Steroid-Refractory Acute Graft Versus Host Disease Final module of rolling Biologic License Application (BLA) submission scheduled for filing with the US Food and Drug Administration (FDA) in January 2020 MPC-06-ID for Chronic Low Back Pain Patient follow up continues through 24-month assessment of safety and efficacy in Phase 3 trial for chronic lower back pain due to degenerative disc disease (H1 2020) with readout planned (mid-2020) Revascor for Advanced Heart Failure In December 2019, surpassed the number of primary endpoint events required for trial completion in the Phase 3 trial of Revascor for advanced heart failure Data read-out for this Phase 3 trial expected by mid-2020 Establish global and/or regional partnerships In advanced discussions on potential blockbuster products1 Mesoblast does not make any representation or give any assurance that such a partnering transaction will be concluded. |

Thank you
